
Ulcerative colitis (UC) is a chronic, relapsing inflammatory bowel disease characterized by mucosal inflammation, dysbiosis, and impaired immune regulation. Conventional management often includes long-term corticosteroid therapy, which can mask autoimmune activity but does not address underlying metabolic and nutritional dysfunctions.
We present the case of a male patient who enrolled in a functional nutrition program for management of UC. Baseline laboratory investigations revealed abnormalities in hematological, metabolic, renal, and hepatic markers, alongside thyroid dysfunction and elevated inflammatory indices. A Gastrointestinal Microbial Assay Plus (GI-MAP) demonstrated severe gut dysbiosis with overgrowth of pathogenic and autoimmune
associated bacteria, markedly elevated calprotectin, eosinophil activation protein, and positive fecal occult blood, consistent with active UC.
The patient was placed on an individualized nutrition protocol that eliminated wheat, dairy, refined sugar, and processed foods and was supported with a phased supplementation strategy including vitamin D3+K2, magnesium, zinc, omega-3 krill oil, probiotics, lactoferrin, glutathione, and colostrum. Over the course of the intervention, progressive improvements were observed in blood parameters, thyroid profile, and gastrointestinal function, along with normalization of key inflammatory markers.
Clinically, the patient reported resolution of rectal bleeding, improved food tolerance, normalization of bowel habits, and enhanced energy levels, enabling resumption of normal professional and personal activities.
This case highlights the potential role of individualized nutrition and targeted supplementation in reducing intestinal inflammation, restoring gut microbial balance, and supporting clinical remission in UC.
Keywords: Ulcerative colitis, Inflammatory bowel disease, Gut dysbiosis
Ulcerative colitis (UC) is a chronic, relapsing inflammatory bowel disease (IBD) characterized by continuous inflammation of the colonic mucosa, beginning in the rectum and extending proximally in a variable pattern. (1) The condition commonly presents with symptoms such as abdominal pain, diarrhea, rectal bleeding, urgency, mucus in stool, and fatigue, which significantly affect quality of life. Unlike Crohn’s disease, ulcerative colitis is confined to the colon and rectum, where it causes continuous inflammation and ulcer formation in the colonic mucosa. (2)
The pathophysiology of UC is complex and multifactorial, involving an inappropriate immune response to gut microbiota in genetically susceptible individuals. Key mechanisms include disruption of the intestinal epithelial barrier, dysbiosis, excessive production of pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β), and oxidative stress. (3) Despite progress in understanding UC, important unmet needs remain. Many patients experience incomplete remission, medication intolerance, side effects, or relapse. Moreover, conventional therapies often focus on symptom suppression rather than addressing underlying triggers such as diet, microbiome imbalance, or systemic inflammation.
The conventional medical approach to UC includes the use of aminosalicylates, corticosteroids, immunomodulators, and biologics aimed at reducing inflammation and maintaining remission. While these therapies can be effective, they may lead to adverse effects, dependency, or diminished efficacy over time.(4)
In contrast, a functional nutrition approach seeks to identify and address root causes and modifiable lifestyle factors contributing to disease progression. This includes optimizing anti-inflammatory dietary patterns, correcting nutrient deficiencies (e.g., vitamin D, iron, omega-3s), supporting gut barrier integrity, and promoting microbiome diversity through prebiotics, probiotics, and individualized food plans.(5) By integrating evidence-based nutrition strategies with conventional care, functional nutrition offers a more holistic framework to improve patient outcomes and long-term disease management.
A 37-year-old male presented to the Functional Nutritionist at Thrivetribe Wellness Solutions Private Limited, Pune with complaints of hematochezia, hemorrhoids, recurrent stomach infections, persistent tiredness, weight gain, nail-biting, and chronic sinusitis. His medical history revealed a diagnosis of ulcerative colitis for which he was receiving infliximab injections, and he was also using steroid inhalers/pumps for the management of sinusitis. His social history was significant for alcohol consumption approximately twice per week and smoking about five cigarettes per week. He reported having adopted a vegetarian diet for the past 4–5 years.
Despite ongoing pharmacotherapy, he continued to experience significant challenges in both physical vitality and mental clarity, limiting his ability to fully engage in work and personal life. Recognizing these unmet needs, he sought a personalized, root-cause–driven nutritional intervention with the goal of reducing symptom burden, supporting gut and immune health, improving energy balance, and restoring overall functional capacity and quality of life.
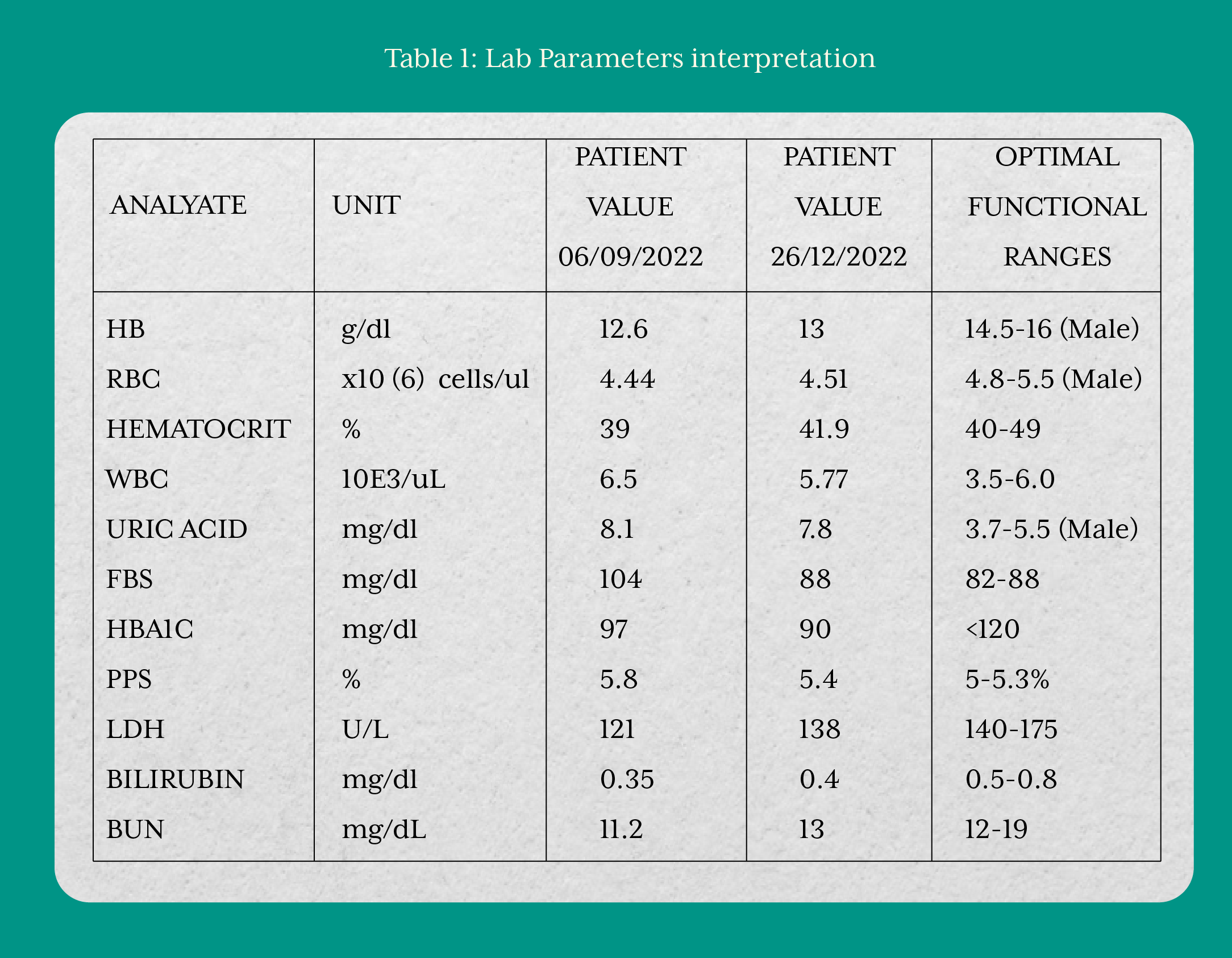
On September 1, 2022, the patient enrolled in the Functional Nutrition Program at Thrivetribe Wellness Solutions Private Limited and provided written informed consent to initiate the intervention. A comprehensive laboratory evaluation performed on September 6, 2022, revealed significant derangements in hematological and metabolic parameters. Red blood cells (RBC) count, hemoglobin, hematocrit, and white blood cells (WBC) levels were abnormal, indicating impaired hematopoietic and immune function. Glycemic markers including fasting blood sugar, hemoglobin A1c (HbA1c), and postprandial sugar were elevated, reflecting poor glucose regulation. Renal (BUN) and hepatic (bilirubin) markers were disturbed, suggesting compromised detoxification and excretory function. Thyroid dysfunction, along with elevated markers of chronic infection and systemic inflammation, were also evident. As the patient had been on long-term steroid therapy, autoimmune status could not be fully established from laboratory investigations alone, warranting further evaluation.
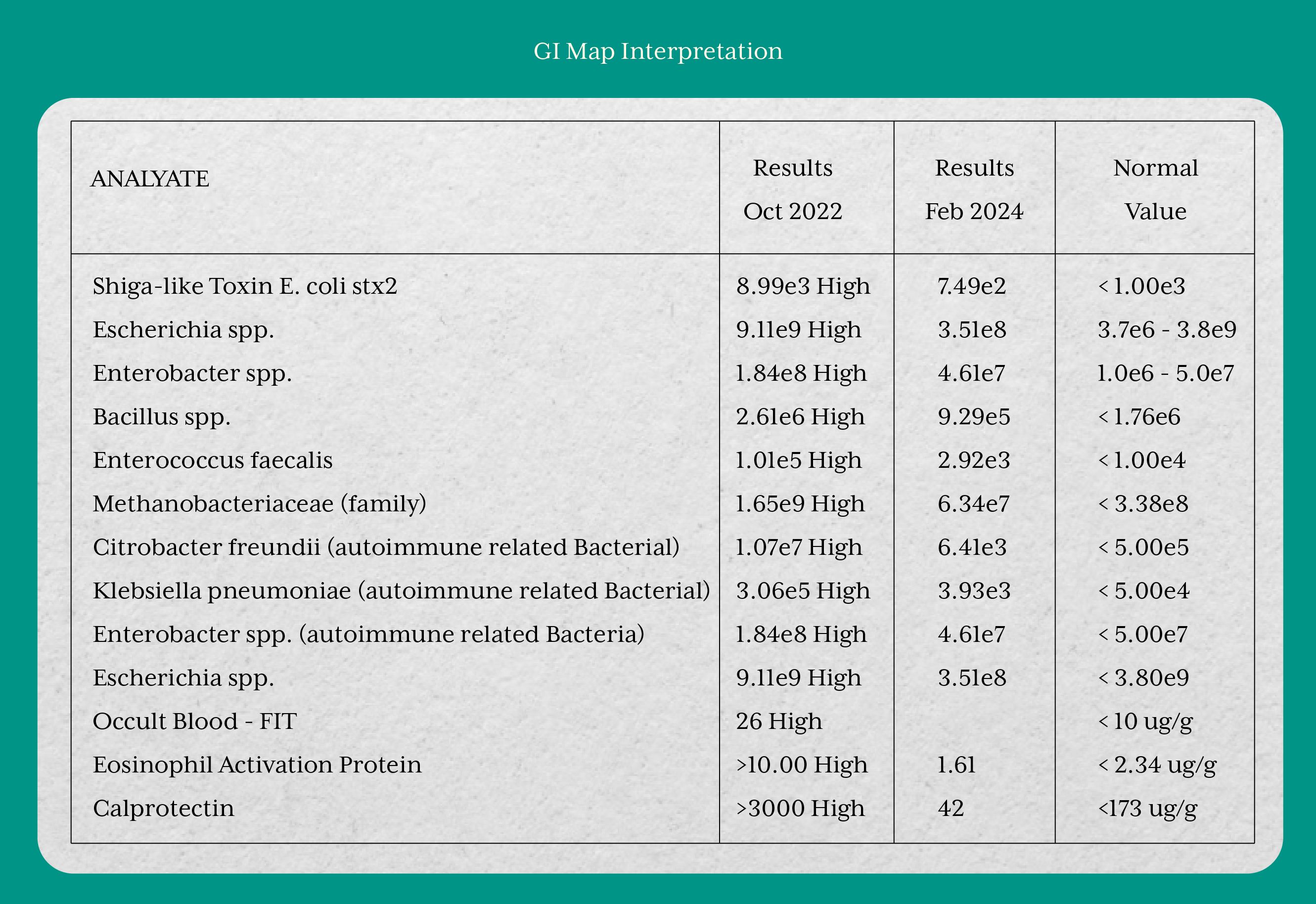
In October 2022, the GI-MAP revealed pronounced gut dysbiosis with overgrowth of several pathogenic and autoimmune-associated bacteria. Inflammatory markers were markedly elevated, with calprotectin exceeding 3000 µg/g, eosinophil activation protein >10 µg/g, and a positive fecal occult blood test, all consistent with severe intestinal inflammation and active mucosal damage in ulcerative colitis.
In light of these findings, an individualized nutrition intervention was initiated together with essential supplementation, aimed at supporting mucosal healing and immune regulation in ulcerative colitis. Dietary modification involved the elimination of wheat and wheat products (to reduce gluten and glyphosate exposure), dairy, refined sugar, and all processed or ultra-processed foods. As the patient was initially restricted to soft foods, a foundational supplement protocol was prescribed, consisting of magnesium bisglycinate (440 mg), vitamin D3+K2 (600 IU + 33.34 mcg), a B-complex, zinc glycinate (elemental zinc 15 mg), probiotics, krill oil, and kelp (600 mg). After 15 days, the patient demonstrated improved food tolerance, allowing for progression to a comprehensive, structured, and phased nutrition protocol.

Phase 1 (Foundational Support): Core supplements were initiated to stabilize gastrointestinal function and reduce inflammation. These included magnesium, vitamin D3+K2, zinc, B-complex, omega-3 krill oil, probiotics, glutathione, lactoferrin, and GI-targeted formulations (GI Soothe, GI Detox, digestive enzymes). An immune support complex and parasite cleanse were also introduced during this phase.
Phase 2 (Targeted Modulation): With symptomatic improvement, additional targeted interventions were added. Pendulum Akkermansia and multi-strain probiotics were prescribed to restore microbial diversity, while “Bye Pylori” was introduced for pathogen modulation. Digestive enzymes and GI Detox were continued to support gut function and toxin clearance.
Phase 3 (Mucosal Repair and Microbiome Support): The final phase was primarily focused on eradicating fungal and mold infections while promoting mucosal healing. Colostrum, Microbiome Support 3, and other foundational supplements were incorporated to enhance epithelial integrity, immunoglobulin support, and restore systemic balance.
Over the course of the intervention, the patient demonstrated relative improvement across hematological, biochemical, and gastrointestinal parameters. Markers of blood glucose regulation, thyroid function, and liver function showed favorable changes. The GI-MAP results also reflected substantial improvement, with a reduction in pathogenic and autoimmune-associated bacterial overgrowth, normalization of inflammatory markers such as calprotectin and eosinophil activation protein, and resolution of occult blood. Collectively, these findings indicated restoration of microbial balance, reduction of intestinal inflammation, and progression toward mucosal healing in ulcerative colitis.
Clinically, the patient reported resolution of gastrointestinal discomfort, absence of rectal bleeding, improved tolerance to a wider range of foods, and restoration of normal bowel habits. He also experienced a marked improvement in overall energy and wellbeing, which enabled him to resume daily activities with greater functional capacity.
The outcomes of this case demonstrate that a phased, nutrition-centered intervention can support clinical and biochemical improvements in ulcerative colitis (UC). Compared with findings in the published literature, several parallels and contrasts emerge.
Our phased dietary strategy, which focused on the elimination of wheat, dairy, refined sugars, and processed foods, combined with targeted supplementation, aligns with functional nutrition principles aimed at restoring gut health. Evidence from clinical studies (Scheller et al., 2019) suggests that structured nutrition programs can support gut barrier integrity and reduce gastrointestinal inflammation. In our patient, this approach was associated with improved dietary tolerance, signs of mucosal healing, and resolution of gastrointestinal symptoms, indicating that individualized functional nutrition strategies may replicate benefits observed in broader clinical models.(6)
Evidence from Radziszewska et al. (2022) suggests that omega-3 fatty acids and probiotics may offer therapeutic benefits in managing ulcerative colitis (UC), though the effects can vary among individuals. In our patient, the initial supplementation phase included omega-3 krill oil and probiotics, leading to a significant improvement in gastrointestinal health. This was evidenced by a reduction in fecal calprotectin levels from >3000 µg/g to 42 µg/g, indicating decreased intestinal inflammation. These outcomes align with the proposed mechanisms of action, supporting the role of targeted supplementation in modulating gut dysbiosis and enhancing mucosal healing in UC.(7)
Sachan et al. (2024) reported that UC patients often present with multiple micronutrient deficiencies, including vitamin D, zinc, and B vitamins, which negatively impact disease activity and quality of life. Our patient similarly demonstrated low vitamin D and zinc levels at baseline, which improved after supplementation. Clinically, this corresponded with significant relief in fatigue, oral ulcers, and recurrent infections, outcomes that align closely with the improvements in functional status described in the prospective cohort study.(8)
Shin and Lim (2020) reviewed the role of functional foods and nutraceuticals in inflammatory bowel disease, emphasizing their capacity to modulate inflammatory pathways, reduce oxidative stress, and support mucosal healing. Specifically, bioactive compounds such as omega-3 fatty acids, polyphenols, and bioactive peptides were highlighted for their ability to regulate cytokine activity and maintain epithelial barrier function. In our case, a similar approach was adopted through elimination of pro-inflammatory foods (gluten, dairy, refined sugar, ultra-processed items) and incorporation of targeted nutraceuticals including krill oil, probiotics, glutathione, and colostrum. The patient’s improved mucosal health and symptomatic remission provide clinical support to the mechanistic framework proposed by Shin and Lim, demonstrating that a structured, functional nutrition protocol can be successfully translated into practice.(9)
In line with these studies, our patient’s case illustrates that combining diet elimination with phased supplementation can lead to improvements in inflammatory biomarkers, gut microbial balance, and clinical quality of life. Unlike many interventional studies, however, our report highlights the feasibility of tailoring such a protocol within a functional nutrition framework, demonstrating that measurable benefits can be achieved even outside controlled trial settings.
The present case demonstrates that targeted nutritional interventions, aimed at modulating inflammation, restoring gut barrier integrity, and rebalancing the microbiome, may play a pivotal role in ulcerative colitis management. Functional nutrition strategies not only contributed to symptom remission but also reduced dependency on conventional medications. This report emphasizes the value of personalized, integrative approaches in chronic inflammatory diseases.









