Introduction
Type 2 diabetes is often presented as a condition that appears abruptly, usually when fasting glucose or HbA1c crosses a diagnostic threshold. In reality, this label arrives very late in the biological timeline. Long before blood sugar rises, long before medication begins, the body has already been struggling with insulin resistance, impaired energy metabolism, and progressive cellular stress.
In India, where metabolic health challenges are rising rapidly, this delay in recognition has serious consequences. Insulin resistance develops silently over years, sometimes decades, while conventional screening continues to focus on late markers. By the time type 2 diabetes is diagnosed, the underlying metabolic dysfunction is already well established.
Understanding insulin resistance as a pre-diagnostic state changes how we view prevention, intervention, and long-term outcomes. It shifts the conversation from sugar management to cellular health.
Understanding Insulin Resistance Beyond Blood Sugar
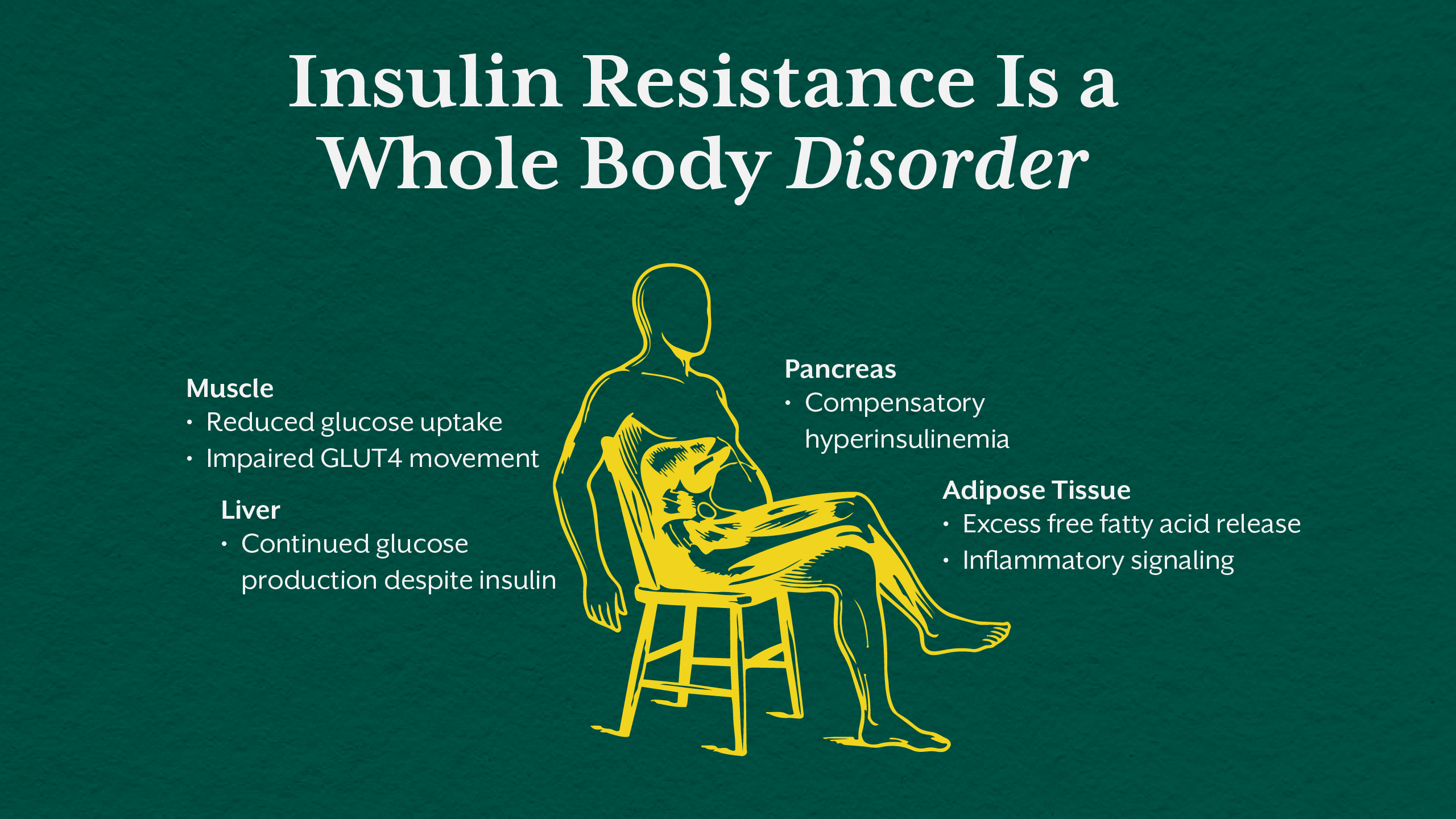
Insulin Resistance Is a Multi-Tissue Disorder
Insulin resistance is not confined to one organ. It is a systemic phenomenon involving skeletal muscle, liver, adipose tissue, and eventually the pancreas.
In skeletal muscle, insulin resistance reduces glucose uptake by impairing GLUT4 translocation. Muscles become metabolically inflexible, unable to efficiently use glucose or fat for energy. In the liver, insulin loses its ability to suppress gluconeogenesis, resulting in excessive glucose output even in the fed state. Adipose tissue becomes dysfunctional, releasing excess free fatty acids and inflammatory signals that further worsen insulin signaling.
These tissue level failures occur long before fasting glucose becomes abnormal. This is why fasting insulin is often a more sensitive early marker than fasting glucose in identifying metabolic dysfunction.
The Role of Mitochondria in Insulin Resistance
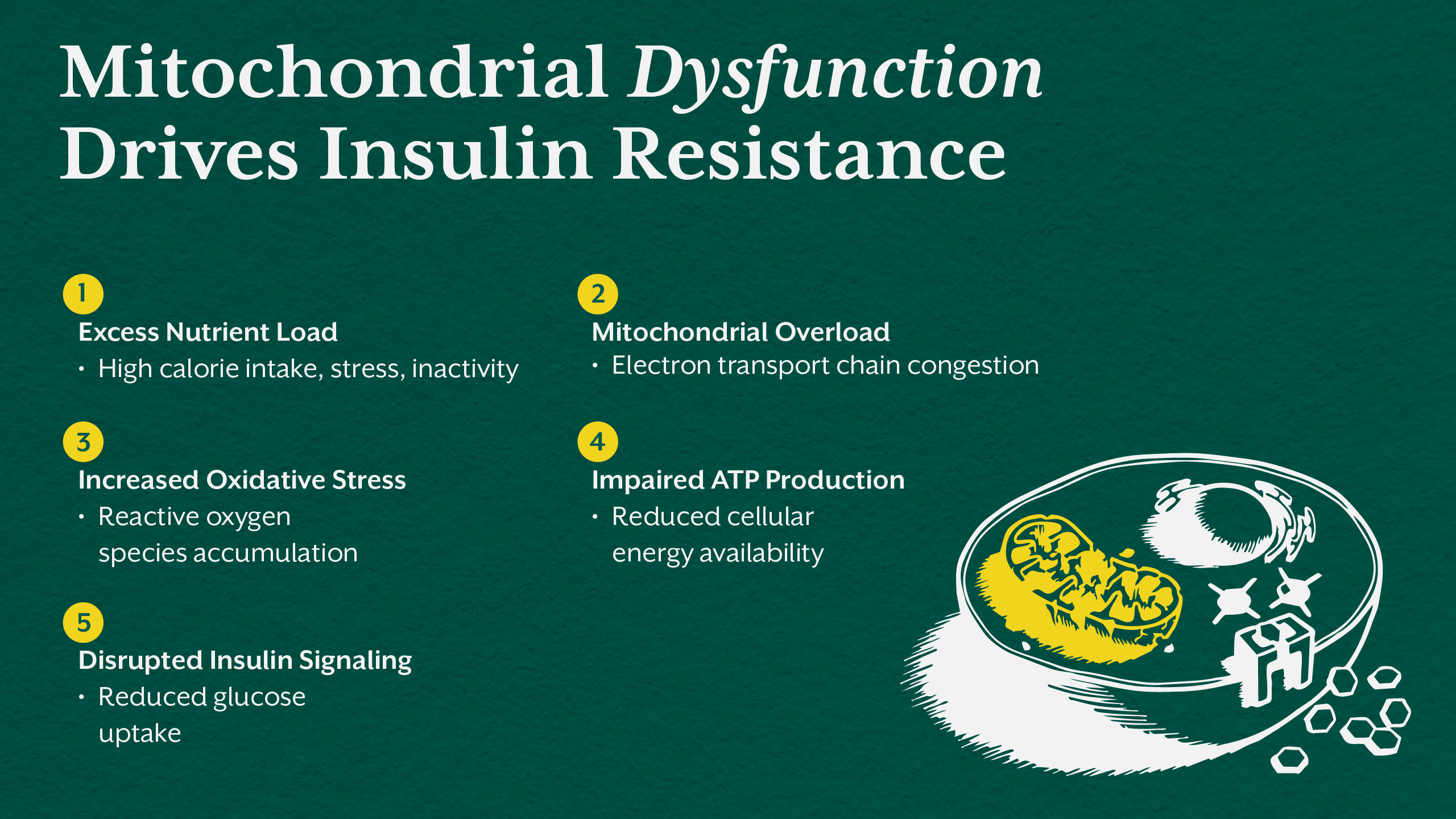
Energy Failure at the Cellular Level
At its core, insulin resistance reflects a failure of energy handling. Healthy cells efficiently convert glucose into ATP through glycolysis, the Krebs cycle, and the electron transport chain. When this system is overwhelmed or impaired, metabolic chaos begins.
Excess caloric intake, sedentary behavior, nutrient deficiencies, chronic stress, and environmental toxins place continuous pressure on mitochondria. When the electron transport chain cannot keep up with substrate flow, electrons back up, increasing reactive oxygen species and oxidative stress.
This oxidative stress damages mitochondrial membranes, impairs ATP synthesis, and disrupts insulin signaling pathways. The result is reduced glucose uptake, rising insulin levels, and progressive insulin resistance. This process explains why type 2 diabetes is fundamentally a disease of energy metabolism rather than simply elevated blood sugar.
If you want to explore how systemic metabolic flexibility affects fat metabolism and insulin performance, our blog The Functional Nutrition Secret to Fat Burning That Mainstream Diets Don’t Want You to Know offers additional insight and context.
Insulin Resistance Precedes Type 2 Diabetes by Years
Why HbA1c Misses the Early Disease Window
HbA1c reflects average blood glucose over the previous two to three months. It is a lagging indicator. By the time HbA1c rises, insulin resistance has already been present for years, often accompanied by hyperinsulinemia, ectopic fat accumulation, and mitochondrial dysfunction.
Fasting insulin, postprandial insulin, and C peptide provide earlier insight into metabolic strain. Elevated fasting insulin signals that the pancreas is compensating aggressively to maintain normal glucose levels. This compensation masks dysfunction until beta cells begin to fail.
In metabolic health India, where carbohydrate heavy diets, chronic stress, disrupted sleep, and physical inactivity are common, reliance on glucose alone delays meaningful intervention.
For a deeper look at how nutritional patterns contribute to early metabolic strain and insulin resistance, you may also find value in reading Top Nutritional Mistakes That Damage Heart Health After 30 on iThrive Academy.
The Twin Cycle Hypothesis and Metabolic Collapse
How Fat Accumulation Drives Disease Progression
The twin cycle hypothesis explains how insulin resistance progresses into overt type 2 diabetes. Excess calories lead to fat accumulation in the liver, causing hepatic insulin resistance and increased glucose production. This excess glucose stimulates insulin secretion, promoting fat storage elsewhere, including the pancreas.
As fat accumulates within pancreatic beta cells, insulin secretion becomes impaired. This creates a vicious cycle of worsening insulin resistance and declining insulin production. Importantly, this process is reversible in its early stages, before irreversible beta cell loss occurs.
Recognizing insulin resistance early allows for intervention before this metabolic tipping point is reached.
Stress, Circadian Disruption, and Inflammation
Modern Triggers That Accelerate Insulin Resistance
Chronic psychological stress activates the HPA axis, increasing cortisol production. Cortisol raises blood glucose through gluconeogenesis and directly interferes with insulin signaling in peripheral tissues. Persistent stress therefore drives both hyperglycemia and insulin resistance.
Exposure to blue light at night suppresses melatonin and disrupts circadian rhythms. This misalignment alters glucose metabolism, increases insulin resistance, and impairs mitochondrial repair processes that normally occur during sleep.
Low grade inflammation, often driven by gut dysbiosis and endotoxin exposure, further blocks insulin signaling. Cytokines such as TNF alpha and IL-6 interfere with insulin receptor function, linking gut health directly to metabolic outcomes.
Why Type 2 Diabetes Is a Late Clinical Label
The Diagnostic Delay Problem
Type 2 diabetes is not the beginning of metabolic disease. It is the point at which compensation fails. The pancreas can no longer produce enough insulin to overcome resistance, and blood glucose finally rises.
By labeling disease at this late stage, healthcare systems miss the opportunity for early metabolic correction. The focus shifts toward glucose lowering rather than restoring insulin sensitivity and cellular energy production.
This framing reinforces the myth that type 2 diabetes is inevitably progressive, when in reality it represents a potentially reversible metabolic state if addressed early.
.webp)
Functional Markers That Reveal Early Metabolic Dysfunction
Looking Beyond Conventional Labs
A functional approach prioritizes early indicators of metabolic strain. These include fasting insulin, postprandial insulin, fasting glucose, postprandial glucose, and C peptide. Together, these markers reveal whether the body is compensating or decompensating.
Advanced testing such as organic acids can highlight mitochondrial bottlenecks, oxidative stress, and nutrient deficiencies affecting the Krebs cycle. Elevated lactate to pyruvate ratios signal electron backup and impaired oxidative phosphorylation. Markers such as adipic and suberic acid indicate poor fatty acid oxidation.
This deeper assessment allows clinicians to identify root causes rather than managing surface level symptoms.
Nutrient Deficiencies and Metabolic Inflexibility
The Biochemical Cost of Modern Diets
Many individuals with insulin resistance are deficient in key micronutrients required for glucose metabolism. Magnesium is essential for insulin receptor signaling and glycolytic enzymes. B vitamins such as thiamine, riboflavin, niacin, and pantothenic acid are required for pyruvate dehydrogenase and the Krebs cycle.
Without these cofactors, glucose cannot be efficiently oxidized, increasing reliance on anaerobic pathways and worsening insulin resistance. Addressing nutrient deficiencies is therefore foundational to restoring metabolic flexibility.
Reframing Intervention: From Management to Metabolic Repair
Early Action Changes Outcomes
When insulin resistance is addressed early, before significant beta cell damage occurs, metabolic reversal becomes possible. Caloric reduction, dietary restructuring, improved sleep, stress regulation, and targeted nutritional support can reduce ectopic fat, restore insulin sensitivity, and normalize glucose handling.
This is where functional nutrition and systems biology approaches offer a meaningful advantage. Education that trains practitioners to interpret metabolic markers, understand mitochondrial physiology, and design individualized interventions is essential for improving outcomes.
We at iThrive Academy focus on developing this clinical lens, equipping health professionals to move beyond glucose centric care toward true metabolic restoration.
Key Takeaway
Type 2 diabetes is not a sudden condition. It is the final stage of years of unresolved insulin resistance, mitochondrial stress, and metabolic overload. Long before blood sugar rises, the body compensates by producing more insulin to maintain normal glucose, masking dysfunction until this system begins to fail.
Insulin resistance is fundamentally a disorder of energy metabolism. Impaired mitochondrial function, oxidative stress, ectopic fat accumulation, inflammation, and nutrient deficiencies disrupt insulin signaling across muscle, liver, and adipose tissue. Conventional markers like fasting glucose and HbA1c detect this process only after significant metabolic damage has already occurred.
Viewing insulin resistance as a pre diagnostic state shifts the focus from glucose control to restoring cellular function. Early markers such as fasting insulin and postprandial insulin reveal metabolic strain sooner and allow timely intervention. When addressed early, before beta cell exhaustion, insulin resistance is not only manageable but often reversible.
The future of metabolic care lies in understanding the cell, not just the sugar, and acting before disease is formally labeled.










.jpg)











